| ProGlyProt ID | BC105 |
| Organism Information |
| Organism Name | Bacillus lentus |
| Domain | Bacteria |
| Classification | Family: Bacillaceae
Order: Bacillales
Class: Bacilli (or Firmibacteria)
Division or phylum: "Firmicutes" |
| Taxonomic ID (NCBI) | 1467 |
| Genome Sequence (s) |
| EMBL | |
| Gene Information |
| Gene Name | |
| NCBI Gene ID | |
| Protein Information |
| Protein Name | Subtilisin (SBL)- Cys mutant |
| UniProtKB/SwissProt ID | Â P29600 |
| NCBI RefSeq | |
| EMBL-CDS | |
| UniProtKB Sequence | >sp|P29600|SUBS_BACLE Subtilisin Savinase OS=Bacillus lentus PE=1 SV=1
AQSVPWGISRVQAPAAHNRGLTGSGVKVAVLDTGISTHPDLNIRGGASFVPGEPSTQDGN
GHGTHVAGTIAALNNSIGVLGVAPSAELYAVKVLGASGSGSVSSIAQGLEWAGNNGMHVA
NLSLGSPSPSATLEQAVNSATSRGVLVVAASGNSGAGSISYPARYANAMAVGATDQNNNR
ASFSQYGAGLDIVAPGVNVQSTYPGSTYASLNGTSMATPHVAGAAALVKQKNPSWSNVQI
RNHLKNTATSLGSTNLYGSGLVNAEAATR |
| Sequence length | 269 AA |
| Subcellular Location | Secreted |
| Function | Subtilisin is an extracellular alkaline serine protease. EC = 3.4.21.62 |
| Protein Structure |
| PDB ID | 1JEA |
| Glycosylation Status |
| Glycosylation Type | S (Cys) linked |
| Experimentally Validated Glycosite(s) in Full Length Protein | N62C, S156C, S166C and L217C (Residues that are shown glycosylated in vitro upon mutation from, N, S and L to Cys respectively) |
| Experimentally Validated Glycosite(s ) in Mature Protein | N62C, S156C, S166C and L217C (Residues that are shown glycosylated in vitro upon mutation from, N, S and L to Cys respectively) |
| Glycosite(s) Annotated Protein Sequence | >1JEA:A|PDBID|CHAIN|SEQUENCE
AQSVPWGISRVQAPAAHNRGLTGSGVKVAVLDTGISTHPDLNIRGGASFVPGEPSTQDGNGHGTHVAGTIAALNNSIGVL
GVAPSAELYAVKVLGASGSGSVSSIAQGLEWAGNNGMHVANLSLGSPSPSATLEQAVNSATSRGVLVVAASGNSGAGSIS
YPARYANAMAVGATDQNNNRASFSQYGAGLDIVAPGVNVQSTYPGSTYASLNGTSMATPHVAGAAALVKQKNPSWSNVQI
RNHLKNTATSLGSTNLYGSGLVNAEAATR
This sequence was mutated at four residues to cysteines which were then glycosylated:
>1JEA:A|PDBID|CHAIN|SEQUENCE
AQSVPWGISRVQAPAAHNRGLTGSGVKVAVLDTGISTHPDLNIRGGASFVPGEPSTQDGC*(62)GHGTHVAGTIAALNNSIGVL
GVAPSAELYAVKVLGASGSGSVSSIAQGLEWAGNNGMHVANLSLGSPSPSATLEQAVNSATSRGVLVVAASGNC*(156)GAGSIC*(166)
YPARYANAMAVGATDQNNNRASFSQYGAGLDIVAPGVNVQSTYPGSTYASC*(217)NGTSMATPHVAGAAALVKQKNPSWSNVQI
RNHLKNTATSLGSTNLYGSGLVNAEAATR
|
| Sequence Around Glycosites (21 AA) | VPGEPSTQDGCGHGTHVAGTI
GVLVVAASGNCGAGSISYPAR
ASGNSGAGSICYPARYANAMA
STYPGSTYASCNGTSMATPHV
|
| Glycosite Sequence Logo | seqlogo |
| Glycosite Sequence Logo | 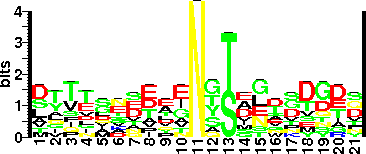 |
| Technique(s) used for Glycosylation Detection | In vitro chemical glycosylation |
| Technique(s) used for Glycosylated Residue(s) Detection | Not applicable |
| Protein Glycosylation- Implication | In vitro engineered glycosylations of native subtilisin has been shown to affect structure as well as enzymatic activity. |
| Glycan Information |
| Glycan Annotation | L217C-S-β-Glc(Ac)2, L217C-S-β-Glc(Ac)3, N62C-S-β-Glc(Ac)4, S156C-S-β-Glc(Ac)4, S16C-S-β-Glc(Ac)4. |
| Technique(s) used for Glycan Identification | Acetylated glycans were predetermined and synthesized in vitro |
| Protein Glycosylation linked (PGL) gene(s) |
| OST Gene Name | |
| OST NCBI Gene ID | |
| OST GenBank Gene Sequence | |
| OST Protein Name | |
| OST UniProtKB/ SwissProt ID | |
| OST NCBI RefSeq | |
| OST EMBL-CDS | |
| OST UniProtKB Sequence | |
| OST EC Number (BRENDA) | |
| OST Genome Context | |
| Characterized Accessory Gene(s) | |
| PGL Additional Links | CAZy |
| Literatures |
| Reference(s) | 1) Davis, B.G., Lloyd, R.C. and Jones, J.B. (2000) Controlled site-selective protein glycosylation for precise glycan structure-catalytic activity relationships. Bioorg Med Chem, 8, 1527-1535. [PubMed: 10976501]
2) Lloyd, R.C. Davis, B.G. and Jones, J.B. (2000) Site-selective glycosylation of subtilisin Bacillus lentus causes dramatic increases in esterase activity. Bioorg Med Chem, 8, 1537-44. [PubMed: 10976502]
3) Graycar, T., Knapp, M., Ganshaw, G., Dauberman, J. and Bott, R. (1999) |
| Additional Comments | Engineered glycoprotein.
Site-selective cysteine (modified mutant) glycosylations of subtilisin, a Bacillus lentus (SBL) protein has been shown to affect structure as well as enzymatic activity post in vitro glycosylation. A positive correlation has also been derived between acetylation of glycan attached and the enzymatic activity and specificity against an esterase substrate succinyl-Ala-Ala-Pro-Phe-S-benzyl by glycosylated subtilisin.
It is one of the first examples of preparations |
| Year of Identification | 2000 |
| Year of Validation | 2000 |


